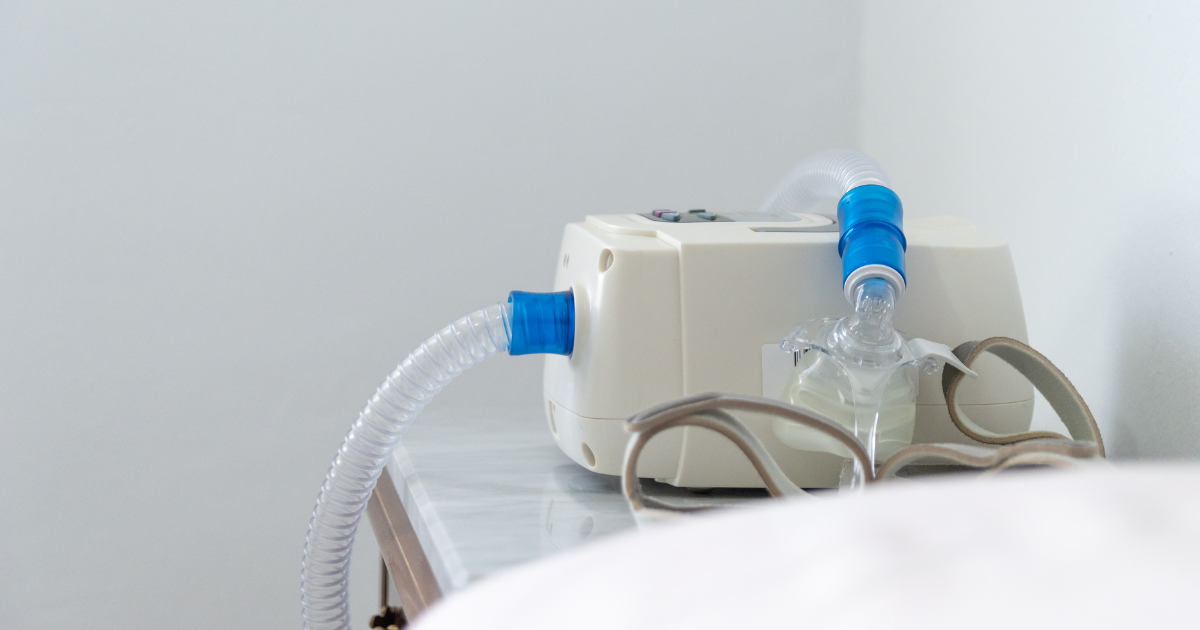
Philips Respironics recently announced that they would be issuing a voluntary recall for continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP) machines. The recall came after it was discovered that the noise-reducing foam that is inside the machines can break down and expose users to cancer-causing toxins. For people who suffer from certain health conditions, like sleep apnea, this is a serious issue. They are put in a position where they must decide between using a device that could make them very sick, or stop using the device and continue suffering the symptoms of sleep apnea, including loud snoring, repeated episodes of stopped breathing and waking up abruptly, and gasping for air.
If you suffered a serious health complication after using a recalled CPAP or BiPAP machine, you may be able to file a products liability lawsuit.
CPAP machines deliver a constant flow of oxygen into a person’s mouth and nose as they sleep. A steady stream of pressurized air flows through a filter and into a flexible tube that connects to a mask that is sealed around the nose and mouth. If you are using a CPAP machine, it is important to understand that the machine does not breathe for you, but it does keep your airways open and helps you to breathe more normally.
The devices use a polyester-based polyurethane (PE-PUR) foam that reduces the noise that the machine makes when it is being used. When the foam breaks down, it can release particles and gases that may cause serious health complications if swallowed or inhaled. In fact, it was found that the degraded foam produced a number of potentially harmful chemicals, including the following:
Philips reported that there were complaints from patients that they found black debris or particles in the device’s airpath circuit. According to a representative from the Food and Drug Administration (FDA), these are serious issues that can have devastating, even life-threatening consequences. In some cases, they can cause permanent impairments. There are a wide range of health complications associated with foam particle inhalation or ingestion, including the following:
When the foam inside the machine breaks down or becomes damaged, it can release volatile organic compounds (VOCs), which are toxic gas emissions. Inhaling these gases causes an increased risk of respiratory complications and cancer. The following are example of health risks associated with inhaling the toxic gases released by PE-PUR foam:
If you have experienced any serious side effects or health complications after using a recalled Phillips CPAP or Bi-PAP device, you may be eligible to pursue a products liability lawsuit. If you suffered any of the following injuries or serious health issues, a lawyer can assist you with the claims process:
The number of products recalled and the problems it has caused is unprecedented. The recall has put pressure on other manufacturers to provide machines to the people who need them. However, there is a limited supply, and users whose device has been recalled are being told that alternative machines are not available. Users are urged to talk to their supplier to determine whether there are other products available that are not impacted by the recall or that may meet their needs until the Philips device is repaired or replaced. Patients should speak with their health care professional about whether they should continue to use the machine.
The following devices have been recalled:
If you are one of the millions of Americans that suffer from sleep apnea, you may rely on a CPAP or BiPAP machine to keep your airways open while you sleep. In addition to helping you breathe normally, a CPAP or BiPAP machine prevents you from waking up multiple times throughout the night, which means you get a better night’s sleep. However, if you discover that the machine you are using has been recalled, it is important that you take the following step to ensure that your health is protected:
If you have been diagnosed with cancer or any other health complication that may be linked to the machine that you have been using, an experienced lawyer can assist you. In order to have a successful outcome, you will need to compile evidence that the device caused your health issue. This may include medical records, test results, the name and model of the device you used, and how long you have been using the device.
Products liability cases involving medical devices can be complicated, so it is highly recommended that you have a skilled lawyer on your side who will protect your legal rights. Depending on the severity of your injuries and other key factors, you may be entitled to the following damages:
If you have suffered a serious health complication after using a recalled CPAP or BiPAP machine, do not hesitate to contact our Los Angeles products liability lawyers at ACTS LAW. Protecting your legal rights is our top priority. To schedule a free, confidential consultation, call us today at 833-228-7529 or contact us online. With offices located in Los Angeles and San Diego, we serve clients throughout Southern California.

When defective and dangerous consumer products cause injuries or even death, it is crucial to hold the responsible parties accountable. Despite consumer protection laws and advanced manufacturing tech...

The National Interagency Fire Center reported a total of 9,280 total wildfires in California in 2021, causing damage to more than 2.3 million acres. The state’s pace has not slowed much in 2022, wit...
A traumatic brain injury (TBI) is as catastrophic as it sounds. It occurs when the brain is struck or jolted from outside forces and often has several debilitating repercussions. According to the Brai...